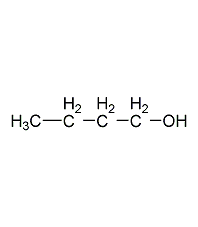
Structural formula
| Business number | 01GF |
|---|---|
| Molecular formula | C4H10O |
| Molecular weight | 74.12 |
| label |
Propanol, Butanol, n-butanol, Chromium alcohol, n-Butyl alcohol, Butyl hydroxide, Butyl alcohol, Aliphatic alcohols, ethers and their derivatives, Raw materials and intermediates used in ink |
Numbering system
CAS number:71-36-3
MDL number:MFCD00002964
EINECS number:200-751-6
RTECS number:EO1400000
BRN number:969148
PubChem number:24892030
Physical property data
1. Properties: colorless and transparent liquid with special smell. [1]
2. Melting point (℃): -89.8[2]
3. Boiling point (℃): 117.7[3]
4. Relative density (water = 1): 0.81[4]
5. Relative vapor Density (air=1): 2.55[5]
6. Saturated vapor pressure (kPa): 0.73 (20℃)[6]
7. Heat of combustion (kJ/mol): -2673.2[7]
8. Critical temperature (℃): 289.85[8]
9. Critical pressure (MPa): 4.414[9]
10. Octanol/water partition coefficient: 0.88 [10]
11. Flash point (℃): 29[11]
12. Ignition temperature (℃): 355 ~365[12]
13. Explosion upper limit (%): 11.3[13]
14. Explosion lower limit ( %): 1.4[14]
15. Solubility: Slightly soluble in water, soluble in most organic solvents such as ethanol and ether. [15]
16. Viscosity (mPa·s, 20ºC): 2.95
17. Heat of evaporation (KJ/mol): 43.86
18. Heat of fusion (KJ/kg): 125.2
19. Heat of formation (KJ/mol): -246.67
20. Specific heat capacity (KJ/(kg· K), 20ºC, constant pressure): 2.33
21. Electrical conductivity (S/m): 9.12×10-9
22. Thermal conductivity Ratio (W/(m·K), 20ºC): 16.75
23. Solubility (%, water, 20ºC): 7.8
24. Volume expansion coefficient (K -1, 20ºC): 0.00095
25. Relative density (20℃, 4℃): 0.8097
26. Relative density (25℃, 4℃): 0.8060
27. Refractive index at room temperature (n25): 1.3971
28. Critical density (g·cm-3 ): 0.271
29. Critical volume (cm3·mol-1): 274
30. Critical compression factor : 0.258
31. Eccentricity factor: 0.595
32. Lennard-Jones parameter (A): 14.00
33. Lennard-Jones parameter (K): 156.3
34. Solubility parameter (J·cm-3)0.5: 23.289
35. van der Waals area (cm2·mol-1): 7.620×109
36. van der Waals volume (cm3·mol-1): 52.400
37. Gas phase standard combustion heat (enthalpy) (kJ·mol-1 ): 2728.22
38. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -274.97
39. Gas phase standard entropy (J·mol-1·K-1): 361.59
40. Gas phase standard formation free energy (kJ·mol-1): -150.0
41. Gas phase standard hot melt (J·mol-1 sup>·K-1): 108.03
42. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -2675.88
43. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -327.31
44. Liquid phase standard entropy (J· mol-1·K-1): 226.4
45. Liquid phase standard formation free energy (kJ·mol-1): -162.72
46. Liquid phase standard hot melt (J·mol-1·K-1): 176.7
Toxicological data
1. Acute toxicity[16]
LD50: 790mg/kg (rat oral); 100mg/kg (mouse oral Oral); 3484mg/kg (rabbit oral); 3400mg/kg (rabbit transdermal)
LC50: 8000ppm (rat inhalation, 4h)
2. Stimulation Sex [17]
Rabbit transdermal: 405mg (24h), moderate irritation.
Rabbit eye: 2 mg, severe irritation.
3. Subacute and chronic toxicity[18] Rats and mice inhaled 0.8mg/m3, 24 hours a week, 4 months, abnormal liver and kidney function.
Ecological data
1. Ecotoxicity[19]
LC50: 1910~1950mg/L (96h) (fathead minnow)
EC50: 2337mg/L (24h), 1983mg/L (48h) (water flea)
IC50: 650mg/ L (72h) (algae)
2. Biodegradability[20]
Aerobic biodegradation (h) : 24~168
Anaerobic biodegradation (h): 96~1296
3. Non-biodegradability [21]
The half-life of photooxidation in water (h): 2602~1.04×105
The half-life of photooxidation in air (h): 8.8~87.7
Molecular structure data
1. Molar refractive index: 22.11
2. Molar volume (cm3/mol): 92.0
3. Isotonic specific volume (90.2K ): 208.0
4. Surface tension (dyne/cm): 26.0
5. Polarizability (10-24cm3): 8.76
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 13.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It forms an azeotropic mixture with water and is miscible with ethanol, ether and other organic solvents. Soluble in alkaloids, camphor, dyes, rubber, ethyl cellulose, resinates (calcium salts, magnesium salts), grease, wax and a variety of natural and synthetic resins.
2. The chemical properties are the same as ethanol and propanol, and it has the chemical reactivity of primary alcohols.
3. Butanol is of low toxicity. The anesthetic effect is stronger than that of propanol, and repeated contact with the skin can cause bleeding and necrosis. It is about three times more toxic to humans than ethanol. Its vapor is irritating to eyes, nose and throat. Even if the concentration is 75.75mg/m3, people will feel unpleasant, but due to its high boiling point and low volatility, it is not dangerous except when used at high temperatures. The oral LD50 in rats is 4.36g/kg. The olfactory threshold concentration is 33.33 mg/m3. TJ 36-79 stipulates that the maximum allowable concentration in workshop air is 200 mg/m3.
4. Stability[22] Stable
5. Incompatible substances[23] Strong acid, acid chloride, acid anhydride, strong oxidizing agent
6. Polymerization hazard[24] No polymerization
Storage method
1. Packed in iron drums, 160kg or 200kg per drum. It should be stored in a dry and ventilated warehouse, the temperature should be kept below 35°C, and the warehouse should be fire-proof and explosion-proof. When loading, unloading and transporting, prevent violent impact and protect it from sun and rain. Store and transport according to regulations on flammable chemicals.
2. Storage precautions [25] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37℃. Keep container tightly sealed. They should be stored separately from oxidants, acids, etc., and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Fermentation method Butanol used to be made from potatoes, grains or sugars as raw materials, and was obtained through hydrolysis and fermentation. The product obtained from the fermentation broth contains approximately 54.8% to 58.5% n-butanol, 30.9% to 33.7% acetone, and 7.8% to 14.2% ethanol. With the development of petrochemical industry, fermentation method is gradually eliminated. The reaction equation is as follows: (C6H10O5)n[n(H2O)]→[Strain]n-C6H12O6[Fermentation]→CH3 COCH3+C4H9OH+C2H5OH
The obtained fermentation broth is then fractionated to obtain acetone, ethanol and n-butanol respectively.
2. The acetaldehyde method uses acetaldehyde as the raw material and adds a dilute alkali solution. The temperature is below 20°C to obtain 2-hydroxybutyraldehyde. When the reaction reaches 50%, it is terminated. The alkali is neutralized with acid and recovered. Unreacted acetaldehyde is extracted from 2-hydroxybutyraldehyde at the bottom of the tower, and then dehydrated using acidic catalysts such as sulfuric acid and acetic acid at 105-137°C to produce crotonaldehyde, and then hydrogenated using a copper complex catalyst at 160-240°C to obtain crude butyraldehyde. Butanol and butyraldehyde are distilled to obtain finished products. CH3CH=CHCHO+H2[catalyst]CH3CH2CH2CHO+CH3CH2CH2CH2OH
3. The propylene carbonylation method uses propylene, carbon monoxide and hydrogen to react on a catalytic bed. The catalyst is zeolite adsorbed cobalt salt or fatty acid cobalt. The reaction temperature is 130~160°C and the reaction pressure is 20~25MPa. The reaction generates n-butyraldehyde and isobutyl aldehyde. Aldehyde is separated through distillation, and n-butyraldehyde is catalytically hydrogenated to obtain n-butanol.
![]()
4. The low-pressure method synthesizes butanol from propylene, carbon monoxide and water in one step. The reaction temperature is 100-104°C and the pressure is 1.5MPa. A mixture of pentacarbonyl iron, n-butylpyrrolidine and water is used. However, the single-pass conversion rate of propylene is low, only 8 %~10%. Reaction equation: CH3CH=CH2+3CO+2H2O→n-C4H 9OH+2CO2
Add acetaldehyde to a dilute alkali solution, react under normal pressure to generate 2-hydroxybutyraldehyde, neutralize the alkali, and then use sulfuric acid and acetic acid Wait for an acidic catalyst to decompose it to obtain crotonaldehyde; then use nickel-chromium as a catalyst and perform a hydrogenation reaction in the presence of excess hydrogen to obtain n-butanol.
5. In the fermentation method, raw materials such as grains, cereals, dried potatoes or molasses are crushed and water is added to make a fermentation liquid. It is sterilized by high-pressure steam and then cooled. Pure acetone-butanol strains are added. Fermented at 36~37℃. The gas produced during fermentation contains carbon dioxide and hydrogen. The fermentation broth contains ethanol, butanol, and acetone, usually in a ratio of 6:3:1. After distillation, butanol, acetone and ethanol can be obtained respectively, or can be used directly as the total solvent without separation.
6. Acetaldehyde condensation method: Acetaldehyde is condensed through aldol to form butaldehyde, which is dehydrated to form crotonaldehyde, and then hydrogenated to obtain n-butanol. ![]()
7. Use industrial n-butanol as Raw materials, add newly calcined calcium oxide, heat and reflux for 4 hours, and filter out the calcium oxide. Add a metal magnesium strip to reflux, then distill under normal pressure, and collect the fraction between 116.5 and 118°C, which is the finished product.
Purpose
1. Mainly used to manufacture n-butyl ester plasticizers of phthalic acid, aliphatic dibasic acid and phosphoric acid, which are widely used in various plastics and rubber products. It is also a raw material for producing butyraldehyde, butyric acid, butylamine and butyl lactate in organic synthesis. It is also a dehydrating agent, anti-emulsifier, extractant for oils, drugs (such as antibiotics, hormones and vitamins) and spices, and an additive for alkyd resin coatings. It can also be used as a solvent and dewaxing agent for organic dyes and printing inks. As a solvent, it can be used to separate potassium perchlorate and sodium perchlorate, as well as sodium chloride and lithium chloride. Used to wash sodium uranyl zinc acetate precipitate. Arsenic acid was determined using the molybdate method in colorimetric determination. Determination of fat in milk. Medium for saponifying esters. Paraffin-embedded material was prepared for microscopic analysis. Used as a solvent for fats, waxes, resins, shellac, gums, etc. Cosolvent for nitrocellulose spray paint, etc.
2. Chromatographic analysis of standard materials. A solvent used for colorimetric determination of arsenic acid and separation of potassium, sodium, lithium and chlorate.
3. Used as analytical reagents, such as solvents and standard materials for chromatographic analysis. Also used in organic synthesis.
4. It is an important solvent and is widely used in the production of urea-formaldehyde resin, cellulose resin, alkyd resin and coatings. It can also be used as a commonly used inactive diluent in adhesives. It is also an important chemical raw material used in the production of plasticizer dibutyl phthalate, aliphatic dibasic acid esters, and phosphate esters. It is also used as a dehydrating agent, anti-emulsifier, extractant for oils, spices, antibiotics, hormones, vitamins, etc., an additive for alkyd resin paints, and a co-solvent for nitrocellulose spray paints.
5. Cosmetic solvents. It is mainly used as a co-solvent in cosmetics such as nail polish to cooperate with main solvents such as ethyl acetate to help dissolve colorants and adjust the evaporation speed and viscosity of the solvent. The addition amount is generally about 10%.
6. It can be used as a defoaming agent for ink preparation in silk screen printing.
7. Used in baked goods, puddings, and candies.
8. Used in the preparation of esters, plastic plasticizers, medicines, spray paints, and as solvents. [26]
extended-reading:https://www.bdmaee.net/n-acetylmorpholine-cas1696-20-4-4-acetylmorpholine/extended-reading:https://www.bdmaee.net/fentacat-11-catalyst-cas63469-23-8-solvay/extended-reading:https://www.bdmaee.net/dimethyltin-dioctanoate/extended-reading:https://www.bdmaee.net/polyurethane-catalyst-9727/extended-reading:https://www.morpholine.org/tertiary-amine-catalyst-dabco-pt303-catalyst-dabco-pt303/extended-reading:https://www.bdmaee.net/dibutyltin-benzoate/extended-reading:https://www.newtopchem.com/archives/39970extended-reading:https://www.morpholine.org/category/morpholine/n-acetylmorpholine/extended-reading:https://www.bdmaee.net/246-trisdimethylaminomethylphenol-cas90-72-2-dabco-tmr-30/extended-reading:https://www.newtopchem.com/archives/703


Comments