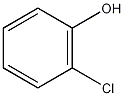
Structural formula
| Business number | 029E |
|---|---|
| Molecular formula | C6H5OCL |
| Molecular weight | 129 |
| label |
o-Chlorophenol, 1-hydroxy-2-chlorobenzene, 1-Hydroxy-2-chlerobenzene, Liquid crystal material intermediates |
Numbering system
CAS number:95-57-8
MDL number:MFCD00002159
EINECS number:202-433-2
RTECS number:SK2625000
BRN number:1905114
PubChem number:24851179
Physical property data
1. Properties: colorless to yellow-brown liquid with unpleasant odor. [1]
2. Melting point (℃): 9.3[2]
3. Boiling point (℃): 174 ~175[3]
4. Relative density (water = 1): 1.26[4]
5. Relative Vapor density (air=1): 4.4[5]
6. Saturated vapor pressure (kPa): 0.13 (12.1℃)[6]
7. Critical pressure (MPa): 5.3[7]
8. Octanol/water partition coefficient: 2.15~2.19[8 ]
9. Flash point (℃): 63.9[9]
10. Explosion limit (%): 8.8 [10]
11. Lower explosion limit (%): 1.7[11]
12. Solubility: easily soluble in water, Soluble in ethanol, ether and sodium hydroxide aqueous solution. [12]
13. Relative density (20℃, 4℃): 1.2634
14. Relative density (25℃, 4℃): 1.2577
15. Refractive index at room temperature (n20): 1.5602d
16. Liquid phase standard hot melt (J· mol-1·K-1): 198.5
Toxicological data
1. Acute toxicity: rat oral LD50: 670mg/kg
2. It is irritating to human skin and eyes, and its dust is also irritating to the human respiratory system. It is a less toxic substance.
3. It is highly irritating and easily absorbed through the skin.
4. Acute toxicity [13]
LD50: 670mg/kg (rat oral)
5. Irritation No information available
6. Mutagenicity[14] Sex chromosome deletion and Without separation, hamster lungs are 800 μmol/L.
7. Others[15] The lowest oral toxic dose in rats (TDLo): 4550mg/kg (70 days before mating/pregnancy 1~21 days) ), affecting the number of fetuses per litter and causing stillbirth.
Ecological data
1. This substance is harmful to the environment and can cause pollution to water bodies and soil, especially to molluscs, fish and mammals. Toxic to aquatic life and can cause adverse consequences to the aquatic environment.
2. Ecotoxicity [16]
LC50: 12.37mg/L (96h) (goldfish, static);
11.63mg/L (96h) (fathead minnow, static);
6.59mg/L (96h) (bluegill, static);
16.7mg/L (48h) (medaka);
2.58mg/L (96h) (water flea)
IC50: 96mg/L (72h) (algae)
3. Biodegradability No data yet
4. Abiotic degradationProperty[17] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, The degradation half-life is 1.6d (theoretical).
5. Bioconcentration[18] BCF: 214 (bluegill sunfish, contact time 28 days); 14~24 (carp , exposure concentration 40ppb, exposure time 6 weeks); 16~29 (carp, exposure concentration 4ppb, exposure time 6 weeks)
Molecular structure data
1. Molar refractive index: 33.02
2. Molar volume (cm3/mol): 99.8
3. Isotonic specific volume (90.2K ): 258.1
4. Surface tension (dyne/cm): 44.7
5. Polarizability (10-24cm3): 13.09
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 3
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 74.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[19] Stable
2. Incompatible substances [20] Acid chloride, strong oxidizing agent, acid anhydride, strong acid
3. Conditions to avoid contact[ 21] Heating
4. Polymerization hazard[22] No polymerization
5. Decomposition products[23] Hydrogen chloride
Storage method
Storage Precautions[24] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. (1) It is obtained by chlorination and acidification of sodium phenolate. Stir the mixture of sodium phenolate, water and ice, slowly add sodium hypochlorite solution below 20°C, control the temperature below 20°C, leave it at room temperature overnight, add concentrated hydrochloric acid while stirring to acidify to pH 2, wash once with water, and then use 5% carbonic acid Wash with the sodium solution until the pH of the wash liquid is 4-5. After cooling, separate the oil layer, carry out atmospheric fractionation, and then distill under reduced pressure to obtain the finished product. (2) Chlorination of phenol with chlorine gas. Add molten phenol to benzene under stirring, and introduce chlorine gas at 26±2°C until the relative density of the chlorinated liquid reaches 0.954 (23-25°C). After removing hydrogen chloride, benzene is steamed out and recovered, steamed to 125°C (21.3kPa), cooled to 60°C, fractionated under reduced pressure, and the 75°C (2.67-3.33kPa) fraction is collected to obtain o-chlorophenol. The chlorination reaction also produces p-chlorophenol and 2,4-dichlorophenol, which are collected as high boilers during fractionation under reduced pressure and can be used as by-products after separation. The yield of o-chlorophenol (more than 95%) is nearly 50%, and the yield of p-chlorophenol (more than 95%) is about 25.5%.
2. O-chlorophenol is a co-product of the production of p-chlorophenol. Chlorinated phenol can obtain ortho- and para-position chlorinated phenols. After separation, p-chlorophenol and o-chlorophenol can be obtained.
Purpose
1. Used for synthesizing pesticides (such as oxalan).
2. Used in organic synthesis. [25]
extended-reading:https://www.bdmaee.net/foaming-retarder-c-225/extended-reading:https://www.bdmaee.net/u-cat-sa-841-catalyst-cas12674-17-3-sanyo-japan/extended-reading:https://www.cyclohexylamine.net/niax-nmm-jeffcat-nmm-lupragen-n105/extended-reading:https://www.newtopchem.com/archives/category/products/page/17extended-reading:https://www.bdmaee.net/wp-content/uploads/2023/02/1-2-1.jpgextended-reading:https://www.newtopchem.com/archives/45044extended-reading:https://www.bdmaee.net/tetramethylpropanediamine-cas110-95-2-tmpda/extended-reading:https://www.newtopchem.com/archives/1830extended-reading:https://www.newtopchem.com/archives/44041extended-reading:https://www.cyclohexylamine.net/reaction-delay-catalyst-polycat-sa-102-delay-catalyst-polycat-sa-102/


Comments