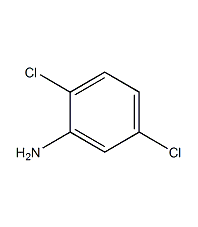
Structural formula
| Business number | 029Y |
|---|---|
| Molecular formula | C6H5Cl2N |
| Molecular weight | 162 |
| label |
Benzene red purple GG salt, Solid Scarlet GG Salt, Fast Scarlet GG Salt, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:95-82-9
MDL number:MFCD00007667
EINECS number:202-455-2
RTECS number:BX2610000
BRN number:1447438
PubChem number:24846562
Physical property data
1. Properties: light brown to amber needle-like crystals. [1]
2. Melting point (℃): 49~51[2]
3. Boiling point (℃) :251[3]
4. Relative density (water = 1): 1.54[4]
5. Relative Vapor density (air = 1): 5.6[5]
6. Octanol/water partition coefficient: 2.75[6]
7. Solubility: Slightly soluble in water, soluble in ethanol, ether, benzene, carbon disulfide, and dilute hydrochloric acid. [7]
Toxicological data
1. Acute toxicity:
Rat oral LD50: 1600mg/kg; rat intraperitoneal LD50: 400mg/kg; mouse oral LD50: 1600mg/kg; mouse intraperitoneal LD50 : 400mg/kg; mouse intravenous LD50: 56mg/kg; rabbit oral LD50: 3750mg/kg; guinea pig oral LD50: 3750mg/kg;
2. Other multiple dose toxicity: rat oral TDLo: 27300mg/kg/13W-I;
3. Acute toxicity[8] LD50: 1600mg/kg (rat oral)
4. Irritation No information available
Ecological data
1. Ecotoxicity No data yet
2. Biodegradability[9] Improved OECD screening test , the degradation is less than 25% in 28 days; in the modified AFNOR test, the degradation is less than 7% in 28 days.
3. Non-biodegradability[10] In the air, when the hydroxyl radical concentration is 5.00×105 pcs/cm3, the degradation half-life is 17h (theoretical).
4. Bioconcentration[11] BCF: 35 (theoretical)
Molecular structure data
1. Molar refractive index: 40.27
2. Molar volume (cm3/mol): 115.6
3. Isotonic specific volume (90.2K ): 304.8
4. Surface tension (dyne/cm): 48.3
5. Polarizability (10-24cm3): 15.96
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 26
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 97.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Uncertain chemical bond configurationNumber of centers: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[12] Stable
2. Incompatible substances[13] Acids, acid chlorides, acid anhydrides, strong oxidants
3. Conditions to avoid contact[14] Heating
4. Polymerization hazard[15] No polymerization
5. Decomposition products[16] Hydrogen chloride
Storage method
Storage Precautions[17] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. The iron powder reduction method uses 2,5-dichloronitrobenzene as raw material, reduces it with iron powder in dilute acid medium, and then neutralizes, separates and refines the finished product.
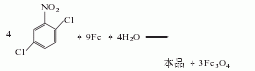
2. Hydrogenation reduction method 2,5-Dichloronitrobenzene is used as the raw material, ethanol is used as the medium and catalyst, and the hydrogenation reaction is carried out under heating and pressure. The reaction product is separated and refined to obtain the finished product.
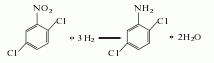
3. Its preparation method is: Add 2,5-dichloronitrobenzene, ethanol and platinum catalyst to the autoclave, introduce hydrogen to 3-4MPa at 85-90°C, then stop flowing the hydrogen and keep it warm. The end point will be reached when the pressure drops stably, and the material Pour it out, remove the solution and crystallize to obtain the product.
Purpose
1. Insoluble azo dyes and ice dyes. Mainly used for cotton dyeing. Coupled with chromophen AS and chromophen AS-D, it dyes bright red; coupled with chromophen AS-G, it dyes light yellow. Strong coupling force and fast coupling speed. It is also used for dyeing viscose fiber, silk and nylon for cotton printing. In addition, it is used to synthesize 2,5-dichlorophenylsuccinic acid.
2. Used as dye intermediate and used in organic synthesis. [18]
extended-reading:https://www.morpholine.org/category/morpholine/page/5398/extended-reading:https://www.morpholine.org/trimethylhydroxyethyl-bisaminoethyl-ether/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/FASCAT2001-catalyst-CAS301-10-0-Stannous-octoate.pdfextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Dioctyltin-dichloride-CAS-3542-36-7-Dioctyl-tin-dichloride.pdfextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/124-2.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2019/10/1-4.jpgextended-reading:https://www.newtopchem.com/archives/44236extended-reading:https://www.newtopchem.com/archives/43976extended-reading:https://www.morpholine.org/polyurethane-blowing-catalyst-blowing-catalyst/extended-reading:https://www.newtopchem.com/archives/44716


Comments