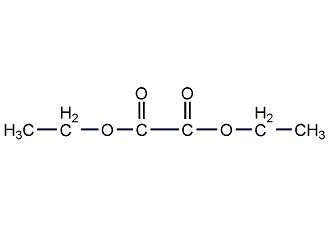
Structural formula
| Business number | 02A5 |
|---|---|
| Molecular formula | C6H10O4 |
| Molecular weight | 146.14 |
| label |
diethyl oxalate, diethyl oxalate, Oxalic acid diethyl ester, Ethyl oxalate, Diethyl ethanedioate, Aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:95-92-1
MDL number:MFCD00009119
EINECS number:202-464-1
RTECS number:RO2800000
BRN number:606350
PubChem number:24848078
Physical property data
1. Properties: colorless oily liquid with aromatic odor. [14]
2. Melting point (℃): -40.6[15]
3. Boiling point (℃): 185.4[16]
4. Relative density (water=1): 1.08 (20℃)[17]
5. Relative vapor density (air = 1): 5.04[18]
6. Saturated vapor pressure (kPa): 1.33 (84℃)[19]
7. Heat of combustion (kJ/mol): -2992.9[20]
8. Critical pressure (MPa): 3.09[21]
9. Octanol/water partition coefficient: 0.56[22]
10. Flash point (℃): 75.6 (CC); 76 (OC) [23]
11. Explosion upper limit (%): 8.4[24]
12. Lower explosion limit (%): 1.5[25]
13. Solubility: miscible in most organic solvents such as ethanol, ether, ethyl acetate, acetone, etc. . [26]
14. Viscosity (mPa·s, 15ºC): 2.311
15. Flash point (ºC, closed): 76
16. Flash point (ºC, open): 75
17. Heat of evaporation (KJ/mol): 41.58
18. Specific heat capacity (KJ/(kg·K ), constant pressure): 1.81
19. Electrical conductivity (S/m, 25ºC): 7.12×10-12
20. Thermal conductivity (W/(m·K), 20ºC): 0.12979
21. Refractive index at room temperature (n25): 1.4074
22. Relative density ( 20℃, 4℃): 1.079
23. Relative density (25℃, 4℃): 1.003186.7
24. Critical density (g· cm-3): 0.33
25. Critical volume (cm3·mol-1): 443 p>
26. Critical compression factor: 0.184
27. Eccentricity factor: 0.568
28. Gas phase standard combustion heat (enthalpy) (kJ·mol-1 ): -3048.2
29. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -742.0
30. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -2984.7
31. Liquid phase standard claimed heat (enthalpy) (kJ·mol- 1): -805.5
32. Liquid phase standard hot melt (J·mol-1·K-1): 264.8
Toxicological data
1. Acute toxicity: Mouse oral LD50: 2000mg/kg; Rat oral LD50: 400~1600mg/kg
2. Mild irritation to the skin. Symptoms of poisoning include respiratory disturbance and muscle tremors, large amounts of oxalic acid deposition in the kidneys, and dilation of renal tubules.
3. Acute toxicity [27] LD50: 400mg/kg (rat oral)
Ecological data
1. Ecotoxicity[28]
LC50: 75mg/L (96h) (fish)
IC50: 7mg/L (72h) (algae)
2. Biodegradability [29] MITI-I test, initial concentration 100mg/L . The sludge concentration is 30 mg/L, and 80% is degraded after 28 days.
3. Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 33.39
2. Molar volume (cm3/mol): 134.5
3. Isotonic specific volume (90.2K ): 320.3
4. Surface tension (dyne/cm): 32.1
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 13.23
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: none
6. Topological molecule polar surface area 52.6
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 114
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[30] Stable
2. Incompatible substances[31] Acids, alkalis, strong oxidants, strong reducing agents, water
3. Conditions to avoid contact [32] Heat
p>
4. Hazards of aggregation[33] No aggregation
Storage method
Storage Precautions[34] Store in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents, acids, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Anhydrous oxalic acid and ethanol are esterified in the presence of the solvent toluene to produce crude diethyl oxalate. The crude ester is distilled into finished products. Raw material consumption quota: oxalic acid 985kg/t, ethanol (95%) 744kg/t, toluene 73.4kg/t.

2. The preparation method is to Add ethanol, benzene and oxalic acid to the reaction kettle and heat to 68°C, azeotropically reflux and dehydrate until no water is brought out as the reaction end point, then recover benzene to obtain crude diethyl oxalate, distill under reduced pressure, and collect the 103°C/6kPa fraction. It is diethyl oxalate.
Refining method: wash with dilute sodium carbonate solution, dry with anhydrous potassium carbonate or sodium sulfate and then distill under reduced pressure.
3. Preparation method:
In a reaction bottle equipped with a stirrer and water separator, add anhydrous oxalic acid ① (2) 45g ( 0.5 mol), 81 g of absolute ethanol (1.76 mol), 200 mL of benzene, and 10 mL of concentrated sulfuric acid. Heat under stirring to reflux and azeotropic dehydration at 68-70°C. After the water is basically evaporated, ethanol and benzene are evaporated. After cooling, wash with water, wash with saturated sodium bicarbonate solution, wash with water, and dry with anhydrous sodium sulfate. Distill under normal pressure and collect the fractions at 182-184°C to obtain 57g of diethyl oxalate (1) with a yield of 78%. Note: ① Anhydrous oxalic acid can be prepared by the following method: Heat the powdered oxalic acid containing crystal water with carbon tetrachloride and reflux, and azeotropically dehydrate until no water evaporates. Filter, dry, and store in a desiccator for later use. Anhydrous oxalic acid can also be prepared by drying it directly in an oven. A corresponding amount of oxalic acid containing crystal water can also be used in this experiment, but the reaction time is longer. [36]
Purpose
1. Diethyl diacetate is mainly used in the pharmaceutical industry, including phenobarbital, azathioprine, sulfonamides, sulfamethoxazole, carboxyl penicillin, ampicillin, chloroquine lactate, Intermediates of thiabendazole and other drugs. It is also a plastic accelerator and dye intermediate. It is also used as a solvent for cellulose esters and spices. Used as acetylene extractant and raw material for dyes, medicines, spices, etc.
2. Diethyl oxalate is often used as a substrate for nucleophiles, and is mostly used for α, γ-dicarbonyl ester [1~3], synthesis of ketone compounds [4~9], heterocyclic compounds [10~13], etc.
Synthesis of α,γ-dicarbonyl ester Under alkaline conditions, ketone compounds can react with oxalic acid Diethyl ester undergoes nucleophilic substitution reaction to generate α,γ-dicarbonyl ester (formula 1)[1,2]. This dicarbonyl ester often exists in the enol structure and can be used to synthesize heterocyclic compounds (Formula 2)[3].

Synthetic ketones
strong> The reaction of diethyl oxalate with Grignard reagent or other organic metal compounds (such as organolithium compounds, etc.) to generate ketones is a good method for preparing α-ester ketones. This method Can be used to synthesize saturated ketones[4,5] (Formula 3) and aromatic ketones[6~9] (Formula 4).
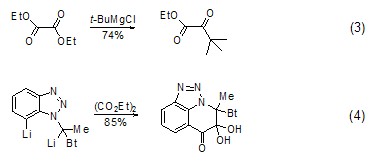
Synthesis of Thiophene Compounds Under alkaline conditions, thiophene derivatives (Formula 5) can be synthesized from diethyl oxalate and β-thioether diester compounds (Formula 5) [10], Selenothiophene derivatives can also be synthesized using similar methods[11].
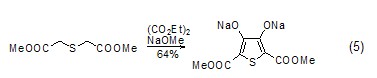
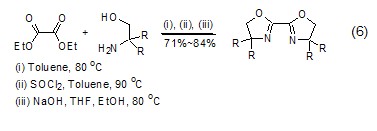
Synthesis of heterocyclic compounds Diethyl oxalate can undergo alcoholysis or aminolysis condensation reaction with compounds with β-hydroxylamine or similar structures to synthesize a heterocyclic structure (Formula 6) [12,13].
3. Used as solvents and dyes Intermediates, and synthesis of paints and drugs. [35]
extended-reading:https://www.bdmaee.net/niax-a-33-catalyst-momentive/extended-reading:https://www.bdmaee.net/bismuth-2-ethylhexanoate-2/extended-reading:https://www.bdmaee.net/dabco-t-catalyst-cas10294-43-5-evonik-germany/extended-reading:https://www.bdmaee.net/cas-26636-01-1/extended-reading:https://www.cyclohexylamine.net/lupragen-dmi-polyurethane-gel-catalyst-polyurethane-gel-catalyst/extended-reading:https://www.bdmaee.net/n-butyltin-hydroxide-oxide/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/N-dimethylaminopropyl-diisopropanolamine-CAS-63469-23-8-PC-CAT-NP10.pdfextended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/2-2.jpgextended-reading:https://www.cyclohexylamine.net/butyltin-trichloridembtl-monobutyltinchloride/extended-reading:https://www.bdmaee.net/dioctyltin-oxide/


Comments