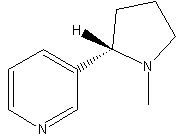
Structural formula
| Business number | 0166 |
|---|---|
| Molecular formula | C10H14N2 |
| Molecular weight | 162.23 |
| label |
1-Methyl-2-(3-pyridyl)pyrrolidine, (S)-(-)-Nicotine, nicotine, (−)-1-Methyl-2-(3-pyridyl)pyrrolidine, nicotine, pesticides |
Numbering system
CAS number:54-11-5
MDL number:MFCD00006369
EINECS number:200-193-3
RTECS number:QS5250000
BRN number:82109
PubChem number:24897663
Physical property data
1. Properties: The pure product is a colorless liquid, odorless 2. Density (g/mL, 25/4℃): 1.0097
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): -80
5. Boiling point (ºC, normal pressure): 246-247oC
6. Boiling point (ºC, 5.2kPa) : Undetermined
7. Refractive index: (n22D) is 1.5282 8. Flash point (ºC): 101
9. Specific rotation (º C=1, DIOXANE): -159.0 ~ -170.0
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): 0.13kPa/61.8℃
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit ( %, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: with water below 60°C Miscible and easily soluble in alcohol, ether, chloroform and petroleum ether.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 49.25
2. Molar volume (cm3/mol): 157.1
3. Isotonic specific volume (90.2K ): 394.2
4. Surface tension (dyne/cm): 39.6
5. Polarizability (10-24cm3): 19.52 p>
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 1.2
2. Hydrogen bondingNumber of donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Tautomers Number:
6. Topological molecular polar surface area (TPSA): 16.1
7. Number of heavy atoms: 12
8. Surface charge: 0
p>
9. Complexity: 147
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 1
12 , The number of uncertain atomic stereocenters: 0
13. The number of determined chemical bond stereocenters: 0
14. The number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Nicotine is a nitrogen-containing alkaline substance that easily reacts with hydrochloric acid to form nicotine hydrochloride, which is soluble in water. Nicotine can be freed by adding strong base NaOH to the extraction solution. Free nicotine has a certain vapor pressure (about 1333Pa) at around 100°C. Therefore, it can be separated and extracted by steam distillation. Nicotine is alkaline and can turn red litmus paper blue and phenolphthalein reagent red. It can be oxidized by KMnO4 solution to generate nicotinic acid, which reacts with alkaloid reagents to produce precipitation.
Storage method
Sealed packaging in brown glass bottles and stored in a dry, ventilated and cool place. Do not store with food or feed. Keep away from children.
Synthesis method
1. There are three industrialized preparation methods for nicotine: distillation, ion exchange resin and extraction. The basic production process is as follows:
The distillation method is to add alkali liquid to tobacco leaves, and then pass superheated steam for distillation. The distilled water contains free nicotine. The ion exchange method is to directly soak tobacco leaves in clean water, filter it to obtain a nicotine aqueous solution, and then flow the aqueous solution through an ion exchange resin column (strongly acidic). The nicotine is enriched on the ion exchange column, and then exchanged with alkali. Obtain an aqueous solution of free nicotine. The extraction method uses lime milk to directly neutralize the nicotine organic acid aqueous solution obtained by soaking tobacco leaves in clean water. The filtered clear liquid is the free nicotine aqueous solution. The nicotine solution obtained by the above method is first extracted with an organic solvent, and then the organic phase is back-extracted with about 30% sulfuric acid aqueous solution. The obtained nicotine sulfate aqueous solution is concentrated to obtain 40% nicotine sulfate. After neutralizing with alkali, excess water is evaporated under normal pressure, and the residue is distilled under reduced pressure to obtain nicotine with a purity of >95%.
2.Nicotine is a major alkaloid in tobacco and has been found in Nicotiana plants of the Solanaceae family. "font-family:Arial;">The main varieties are Nicotiana tabacum and Nicotiana rustica.
1. Made from tobacco - dilute acid impregnation - alkali neutralization - steam distillation - oxalic acid treatment - alkalization.
2. Tobacco leaves - grinding (or using by-product tobacco powder from the cigarette factory) - lime milk alkalization - kerosene filtration.
Purpose
1. This product is a non-residue, non-systemic contact insecticide and has some ovicide activity. Used on rice, fruit trees, and vegetables to control rice miners, rice planthoppers, aphids, leaf rollers, cabbage caterpillars, etc. It can also be used for fumigation of confined spaces (greenhouses, etc.). Using nicotine as a lead compound can also produce new high-efficiency pesticides.
extended-reading:https://www.cyclohexylamine.net/dabco-33-lsi/extended-reading:https://www.morpholine.org/127-08-2/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/18.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/4.jpgextended-reading:https://www.newtopchem.com/archives/1133extended-reading:https://www.bdmaee.net/dabco-ne1070-polyurethane-gel-type-catalyst-dabco-low-odor-catalyst/extended-reading:https://www.bdmaee.net/low-odor-reactive-catalyst/extended-reading:https://www.cyclohexylamine.net/organic-bismuth-catalyst-dabco-mb20-dabco-mb20/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/NEWTOP8.jpgextended-reading:https://www.bdmaee.net/lupragen-n106-strong-foaming-catalyst-di-morpholine-diethyl-ether-basf/


Comments