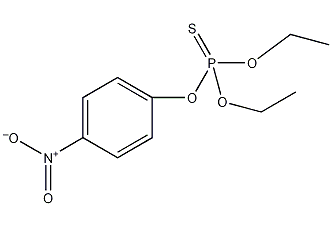
Structural formula
| Business number | 017K |
|---|---|
| Molecular formula | C10H14NO5PS |
| Molecular weight | 291.26 |
| label |
Ethyl 1605, Barasone, Ethyl parathion, O,O-diethyl-O-(4-nitrophenyl)phosphorothioate, Parathion-ethyl, Ethyl parathion, Etilon, O,O-Diethyl-O-(4-nitrophenyl)phosphorothioate, Organophosphorus pesticides, Multifunctional solvent |
Numbering system
CAS number:56-38-2
MDL number:MFCD00036219
EINECS number:200-271-7
RTECS number:TF4550000
BRN number:None
PubChem number:24869108
Physical property data
1. Properties: The pure product is a colorless and odorless liquid, while the industrial product is a brown liquid with a garlic smell. [1]
2. Melting point (℃): 6.0[2]
3. Boiling point (℃): 375 [3]
4. Relative density (water = 1): 1.27[4]
5. Saturated vapor pressure (kPa): 0.08 (157℃) [5]
6. Octanol/water partition coefficient: 3.83[6]
7. Flash point (℃): 174[7]
8. Solubility: insoluble in water, soluble in alcohols, ethers, esters, ketones It contains organic solvents such as hydrocarbons and aromatics, and is insoluble in petroleum ether and kerosene. [8]
9. Refractive index (25ºC): 1.5370
10. Specific rotation (º): c=5, H2O): - 16--200
Toxicological data
1. Acute toxicity[9]
LD50: 6~15mg/kg (rat oral); 5~100mg/kg (Rabbit transdermal)
LC50: 31.5mg/m3 (Rat inhalation, 4h)
2. Irritation No data yet
3. Mutagenicity [10] Microbial mutagenicity: Salmonella typhimurium 1mg/dish. Sister chromatid exchange: human lymphocytes 200μg/L. Unprogrammed DNA synthesis: human fibroblasts 10 μmol/L. DNA damage: rat lymphocytes 10 μmol/L (16h).
4. Carcinogenicity [11] IARC Carcinogenicity Comment: G3, insufficient evidence of carcinogenicity to humans and animals.
5. Others[12] The lowest oral toxic dose in rats (TDLo): 360μg/kg (pregnancy 2~22d/postpartum 15d) , affecting the biochemistry and metabolism of newborn rats. The lowest subcutaneous toxic dose in rats (TDLo): 9800 μg/kg (gestation 7 to 13 days), causing fetal death.
Ecological data
1. Ecotoxicity[13]
LC50: 1.41mg/L (96h) (fathead minnow); 0.065mg/L (96h) (Bluegill); 0.425mg/L (96h) (Green Sunfish); 0.19mg/L (96h) (Largemouth Bass); 0.0008mg/L (24h), 0.00037mg/L (48h) ) (water flea); 0.0006mg/L (48h) (Flea-like flea)
2. Biodegradability No data available
3. Non-biodegradable Properties[14] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 4.2h (theoretical).
4. Bioconcentration [15] BCF: 63~462 (bluegill sunfish, contact concentration 0.51~0.64mg/L, contact time 0.5~3 days); 68~344 (brook char, contact concentration 0.27~3.18mg/L, contact time 0.33~5.83 days)
Molecular structure data
1. Molar refractive index: 70.99
2. Molar volume (cm3/mol) 219.5
3. Isotonic specific volume (90.2K) :591.7
4. Surface tension (dyne/cm): 52.7
5. Polarizability (10-24cm3): 28.14
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 106
7. Number of heavy atoms: 18
8. Surface charge: 0
9. Complexity: 304
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Rapidly hydrolyzes in alkaline media; relatively stable in neutral or slightly acidic solutions; unstable to ultraviolet light and air.
2. Stability[16] Stable
3. Incompatible substances[17] Strong oxidants, alkalis
4. Conditions to avoid contact[18] Heating
5. Polymerization hazard[19] No polymerization
6. Decomposition products[20] Phosphorus oxide, sulfur oxide
Storage method
Storage Precautions[21] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. It is synthesized from diethylphosphorothioate chloride and sodium p-nitrophenolate under the catalysis of trimethylamine. Raw material consumption quota: phosphorus pentasulfide 370kg/t, absolute ethanol 330kg/t, sodium p-nitrophenolate 330kg/t.
2. It is synthesized from diethylphosphorothioate chloride and sodium p-nitrophenolate under the catalysis of trimethylamine.
Purpose
1. Parathion is a broad-spectrum pesticide with contact killing, stomach poisoning, and fumigation effects. It has no systemic conduction effect, but can penetrate into plants. It has a rapid effect on insects, and its insecticidal effect is significantly accelerated at high temperatures. It can be used to control fruit tree pests such as cotton, apples, citrus, pears, peaches, and wheat spider mites.
2. A broad-spectrum, highly toxic insecticide with acaricidal effect, strong contact killing and gastric poisoning effects, a certain fumigation effect, no systemic effect, but a strong penetrating effect . It can control a variety of pests on crops such as rice, cotton and fruit trees. It mainly controls rice borer, cotton bollworm, corn borer, sorghum borer and other pests. Prevent and control three-poled borer, two-poled borer, and giant borer. It is prohibited to use it on vegetables, tea, fruit trees, and Chinese herbal medicines. Melons are sensitive to parathion, especially seedlings, which can easily cause phytotoxicity and should not be used. It is forbidden to eat edible crops 30 days before harvest.
3. Used as pesticides, insecticides and acaricides. [22]
extended-reading:https://www.bdmaee.net/cas-2273-43-0-2/extended-reading:https://www.newtopchem.com/archives/40579extended-reading:https://www.newtopchem.com/archives/1873extended-reading:https://www.bdmaee.net/dioctyltin-dilaurate-dotdl/extended-reading:https://www.bdmaee.net/ethylhexanoic-acid-zinc-salt/extended-reading:https://www.newtopchem.com/archives/45050extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/31-6.jpgextended-reading:https://www.bdmaee.net/nt-cat-la-500-catalyst-cas10861-07-1-newtopchem/extended-reading:https://www.bdmaee.net/fentacat-11-catalyst-cas63469-23-8-solvay/extended-reading:https://www.newtopchem.com/archives/658


Comments