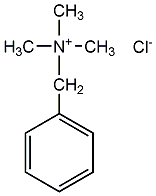
Structural formula
| Business number | 00JU |
|---|---|
| Molecular formula | C10H16ClN |
| Molecular weight | 185.70 |
| label |
N,N,N-trimethylphenylmethylammonium chloride, Trimethylbenzyl ammonium chloride, emulsifier, cellulose solvent, Polymerization inhibitor |
Numbering system
CAS number:56-93-9
MDL number:MFCD00011782
EINECS number:200-300-3
RTECS number:BO8400000
BRN number:3917255
PubChem number:24848426
Physical property data
1. Properties: White or light yellow crystals, easy to absorb moisture.
2. Density (g/mL, 20/4℃): 1.072
3. Melting point (ºC): 239 (decomposition)
4. Refraction Rate (20ºC): 1.470
5. Solubility: Easily soluble in water, ethanol, hot benzene and butanol, slightly soluble in dibutyl phthalate and tributyl phosphate, insoluble in ether. Irritating.
Toxicological data
1. Acute toxicity: Rat oral LDLo: 250mg/kg; mouse oral LDLo: 1600mg/kg
2. Irritating to eyes, respiratory system and skin.
Ecological data
None yet
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 107
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
Hygroscopic. It is stable below 135℃ and decomposes into benzyl chloride and trimethylamine above 135℃. Protective clothing should be worn when using.
Storage method
This product should be sealed and stored in a cool, dry place.
Synthesis method
The mixture of benzyl chloride, trimethylamine and methanol was refluxed for 4 hours, the methanol was evaporated to obtain a crude product, and the finished product benzyltrimethylammonium chloride was obtained through ethanol recrystallization with a yield of 88%.
Purpose
Reagents, emulsifiers, cellulose solvents, and polymerization inhibitors for the determination of platinum, palladium, mercury, and gold.
extended-reading:https://www.newtopchem.com/archives/category/products/page/122extended-reading:https://www.morpholine.org/category/morpholine/page/5404/extended-reading:https://www.newtopchem.com/archives/1002extended-reading:https://www.bdmaee.net/wp-content/uploads/2020/06/67.jpgextended-reading:https://www.newtopchem.com/archives/category/products/page/31extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/FASCAT2001-catalyst-CAS301-10-0-Stannous-octoate.pdfextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/38-2.jpgextended-reading:https://www.newtopchem.com/archives/category/products/page/145extended-reading:https://www.newtopchem.com/archives/748extended-reading:https://www.newtopchem.com/archives/1853


Comments