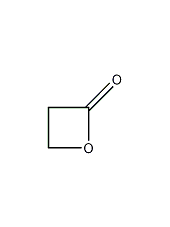
Structural formula
| Business number | 018T |
|---|---|
| Molecular formula | C3H4O2 |
| Molecular weight | 72.06 |
| label |
2-Oxetanone, β-Propionolactone, Propanolide, vaccine inactivator, disinfectant, preservative, fungicides, Standard materials for analysis |
Numbering system
CAS number:57-57-8
MDL number:MFCD00005169
EINECS number:200-340-1
RTECS number:RQ7350000
BRN number:1360
PubChem number:24898684
Physical property data
1. Properties: flammable, colorless and toxic liquid.
2. Density (g/mL, 25/4℃): 1.1460
3. Relative density (g/mL, 20℃): 1.148
4. Melting point (ºC): -33.3
5. Boiling point (ºC, normal pressure): 162 (decomposition)
6. Flash point (ºC): 74 (open cup method)
7. Refractive index (20ºC): 1.4135
8. Flash point (ºC): 70
9. Boiling point (ºC, 6.65KPa): 80
10. Boiling point (ºC, 2.26KPa): 61
11. Boiling point (ºC, 1.33KPa): 51
12. Vapor pressure (Pa, 25ºC): 453.3
13. Relative density (20℃, 4℃): 1.1460
14. Refractive index at room temperature (n25): 1.4117
15. Solubility parameter (J·cm-3)0.5: 29.036
16. van der Waals area (cm 2·mol-1): 4.900×109
17. van der Waals volume (cm 3·mol-1): 35.660
18. Liquid phase standard hot melt (J·mol-1·K-1): 123.1
19. Solubility: soluble in most organic solvents, often in CH2Cl2, Used in THF and DMF.
20. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -1469.3
21. Gas phase standard claimed heat (enthalpy) ( kJ·mol-1): -282.9
22. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -1422.3
23. The liquid phase standard claims heat (enthalpy) (kJ·mol-1): -329.9
Toxicological data
1. Skin irritation: Both liquid and steam are irritating to the skin, and prolonged contact may cause rashes and blisters.
2. Systemic toxicity: mouse intravenous LD50 345±8.8mg/Kg.
When the content of the little white rat inhaled the air in the air is 0.36mg/L, after exposing 2HR, the death is 40%; when the concentration is 0.72 mg/L, the death is 80%after exposed 2HR.
When a dog inhales air with a concentration of 0.45 mg/L, he will die two days after being exposed for 2 hours.
3. Carcinogenicity: Carcinogenic to animals.
4. Strongly irritating to skin, mucous membranes and eyes.
Ecological data
When containing a large amount of polymer, it will settle on the surface of the object after spray disinfection. It is neither volatile nor soluble in water, making it difficult to remove.
Molecular structure data
1. Molar refractive index: 15.57
2. Molar volume (cm3/mol): 58.5
3. Isotonic specific volume (90.2K ): 145.9
4. Surface tension (dyne/cm): 38.7
5. Polarizability (10-24cm3):6.17
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.2
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 57.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable when placed in a glass bottle at 5℃. Hydroxypropionic acid is generated when moisture enters or is hydrolyzed, and its aqueous solution is rapidly decomposed. The hydrolysis rate accelerates with the increase of temperature. The half-life of the aqueous solution is only 18hr at 10°C, 3.5hr at 25°C, 20min at 50°C, and 5min at 75°C. If stored at high temperature, it will polymerize within 6 to 8 weeks. Inorganic salts, acids or bases have a catalytic effect on isopropyl lactone liquid, or react with it to form new products. If stored for too long, the active ingredients should be measured before use.
2. It may have carcinogenic toxicity and is highly volatile, so it is best to use and operate it in a fume hood.
3. Due to its high volatility and toxicity, it is not recommended to prepare it in the laboratory.
4. Found in tobacco leaves.
5. Carcinogenic.
Storage method
Seal and store in a dry place at 4°C.
Synthesis method
1. Ketone is obtained by cracking acetic acid, and then combined with formaldehyde in a molar ratio of 3:1, using chlorine as the diluent, and gas phase condensation or liquid condensation under the action of BF3 catalyst.
2. This product can be synthesized by reacting β-iodopropionic acid with silver oxide.
3. The catalytic carbonylation reaction of propylene oxide and CO can be used to prepare this reagent [1].
Purpose
1. Used as an intermediate for drugs, resins and fiber modifiers, also used as a bactericidal disinfectant, used in plasma and vaccines Sterilize. The derivative β-mercaptopropionic acid is a PVC stabilizer and a raw material for medicine. It is also used as a solvent, organic synthesis intermediate, and analytical standard material.
2. β-Propanolactone is mainly used as a three-carbon synthon in organic synthesis. Depending on the reaction conditions or the nucleophile in the reaction, the reaction can selectively proceed along pathway A or pathway B to generate two different types of products (Formula 1).
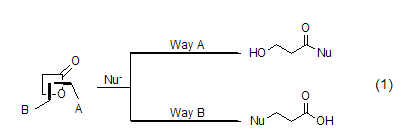
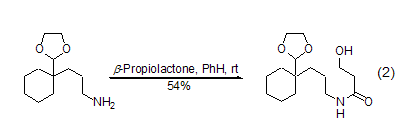
β-Propanolactone and metal nucleophiles (such as lithium reagent and Grignard reagent) Mainly Proceeding along pathway A, there is no obvious difference from the normal reaction. But in most cases, mixtures are generated, and the meaning of synthesis is gradually replaced by other reactions. The most commonly used reaction now is the alcoholysis or amidolysis of β-propionolactone to generate the corresponding β-propanolate or β-propanolate amide (formula 2)[2~4] .
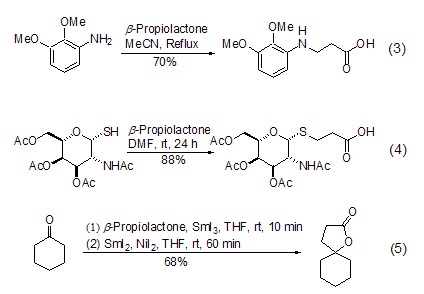
β- Propanolactone and phenol[5], aromatic amines[6,7], thiols[8], ketones or imines [9]The reaction occurs selectively along pathway B. Under these conditions, aromatic amines can easily generate β-aminopropionic acid (Formula 3). Thiol and β- propionolactone form β- thioether propyl Acid reactions are particularly useful in the synthesis of sulfur-containing sugar compounds. The mild reaction conditions hardly have any effect on other functional groups (Formula 4). Ketones or imines will generate cyclic structural compounds, and if they react with cyclic ketones or cyclic ketimines, they will generate products with spirocyclic structures (Formula 5).
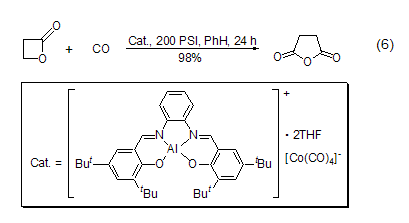
β-Propanolactone reacts with titanocene reagents to generate enol cyclic ethers with exocyclic double bonds[10]. In the presence of metal catalysts Under the condition of [11]
extended-reading:https://www.cyclohexylamine.net/high-quality-cas-100-74-3-n-ethylmorpholine/extended-reading:https://www.newtopchem.com/archives/1755extended-reading:https://www.newtopchem.com/archives/44947extended-reading:https://www.bdmaee.net/wp-content/uploads/2020/06/73.jpgextended-reading:https://www.newtopchem.com/archives/category/products/page/72extended-reading:https://www.cyclohexylamine.net/zinc-neodecanoatecas-27253-29-8/extended-reading:https://www.cyclohexylamine.net/dabco-mp601-delayed-equilibrium-catalyst/extended-reading:https://www.newtopchem.com/archives/category/products/page/53extended-reading:https://www.newtopchem.com/archives/39388extended-reading:https://www.newtopchem.com/archives/40329


Comments